August 23 2018 While GW Pharmaceuticals was conducting the clinical trials that led to FDA approval of Epidiolex for treating two rare forms of severe childhood epilepsy. the company was also backing an “add on” trial of the drug —which is almost pure CBD— for treating all kinds of Treatment Resistant Epilepsy (TRE). Some 30 to 40 percent of all epilepsies are treatment resistant.
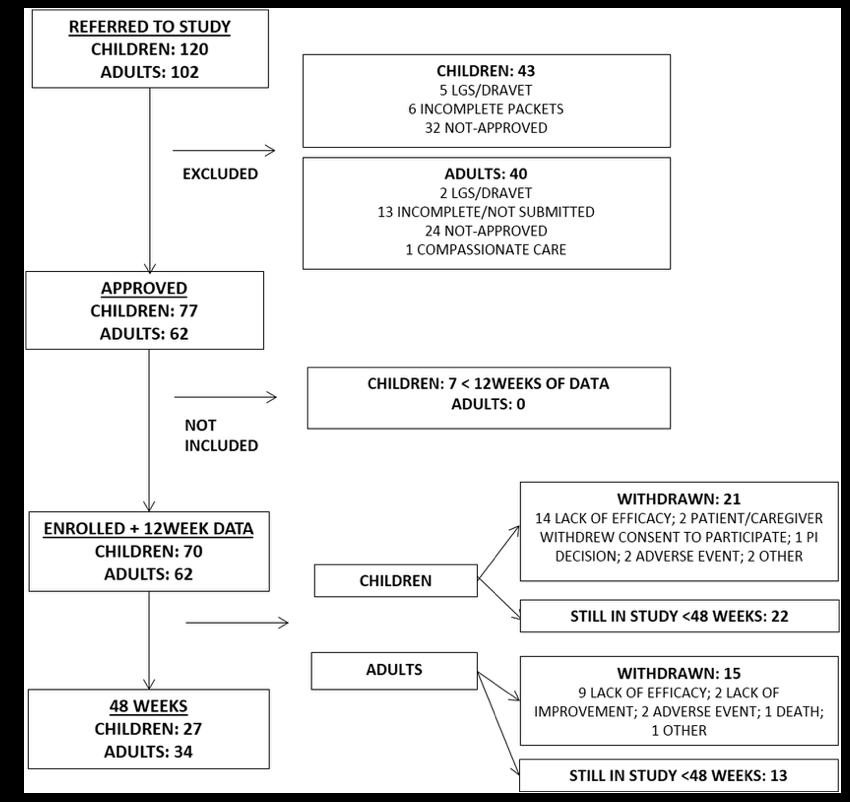
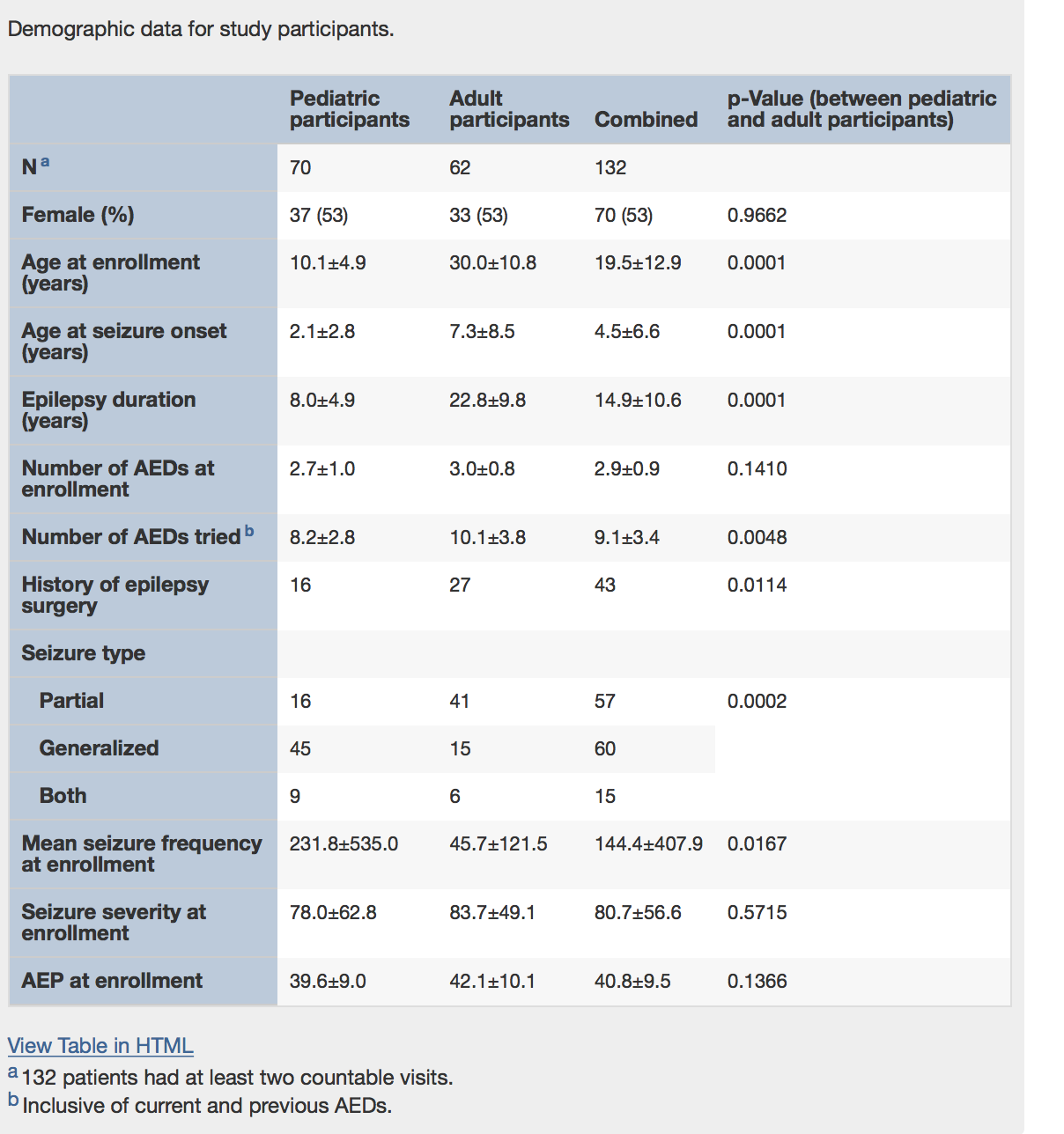
Researchers at the University of Alabama at Birmingham conducted an open-label Extended Access Program safety study, following 132 patients —60 adults, 72 children. The very promising results were just published in Epilepsy & Behavior.
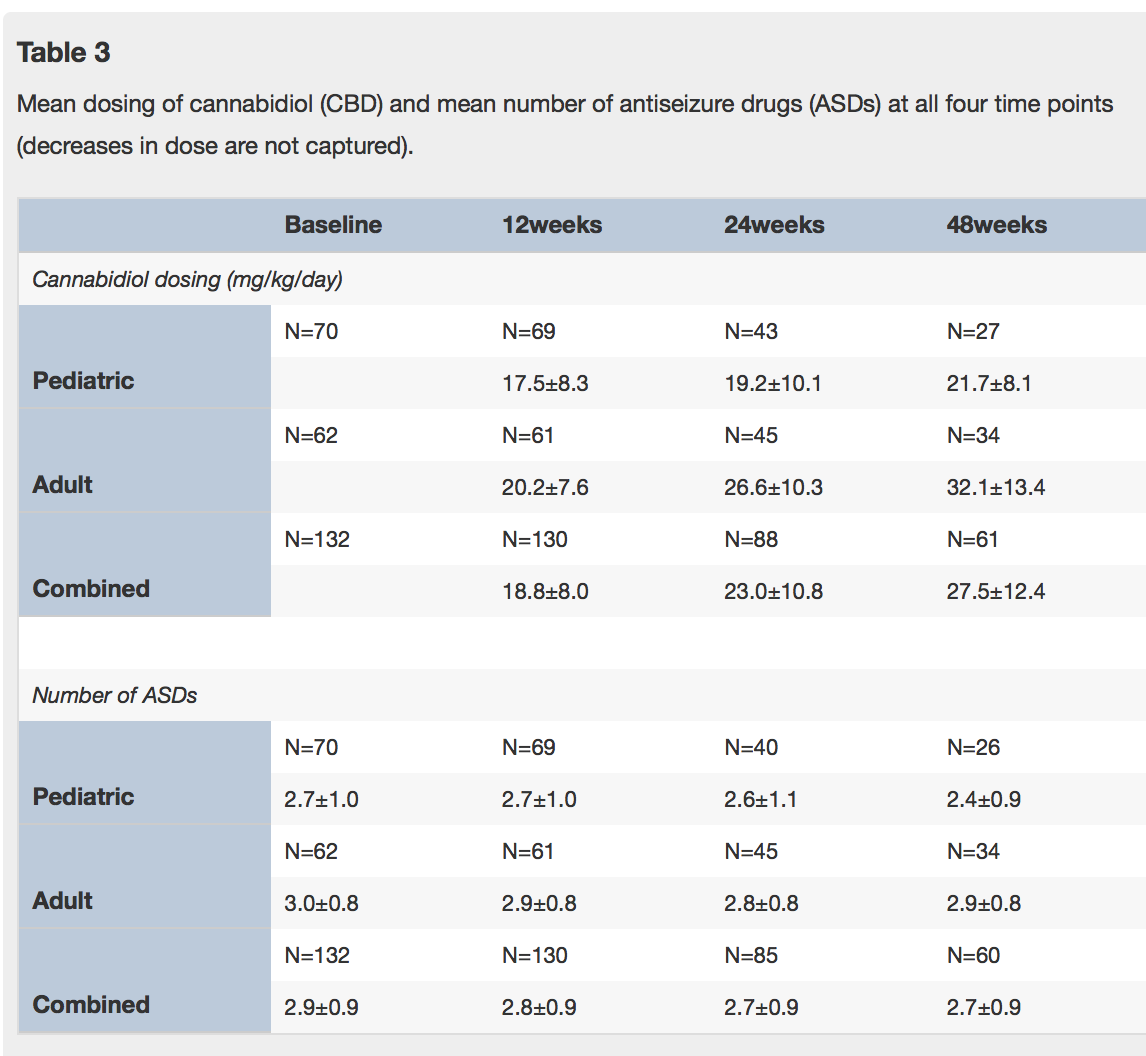
The study retention rate was 77% at one year and adverse events were significantly reduced, as summarized in the tables below: