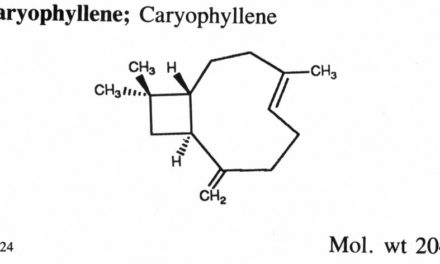
By an FDA Consultant (O’S Spring 2005)
I’ve never heard anyone at a drug company or FDA talk about medical marijuana, but there’s a lot of useful experience with other botanicals.
The first step in qualifying a product for medicinal purposes, here or in any other developed country, is to have a well-defined product, and this is where most botanicals have difficulty. Even a botanical with a strong claim for homogeneity (e.g., one made in huge, well-mixed batches, with some sort of convincing standards to control batch-to-batch variation) would be suspect; it must contain many chemical species, and it’s unlikely that all are useful or even benign.
Why not hold out for a better-defined preparation? Opium and digitalis leaf were both important, but regulators and clinicians feel that they are on firmer ground with morphine, codeine, digoxin, or digitoxin. It might have turned out that morphine works only in the presence of codeine, and so on, but I don’t know of any example where anything like that is the case.
There is no legal basis for FDA recognition of the differential cost of one medication over another, so moderate difficulty in separating the components of a botanical does not elicit any bankable official sympathy.
On the other hand, many so-called ‘pure’ substances are in fact racemic mixtures, and there is only tepid interest in specificity at the enantiomer level. Even returning to botanicals, the heterogeneity issue would be tractable if there were solid proof of safety and efficacy, and if separation of the active components appeared to be extraordinarily difficult. Suppose that a sponsor presented data to that effect to FDA.
That’s the point at which the FDA would start to hear from outsiders in the Administration and in drug companies. The Administration would be able to weigh in first because an application before FDA is not publicly discussed until an (optional) Advisory-Committee meeting, late in the course of review. Before then, the Administration could quietly arrange that the application be rejected. I would have regarded this possibility as far-fetched until this year, but all things are possible with Bush.
The drug companies might chime in at an Advisory-Committee meeting, but it’s hard to imagine a scenario in which things got that far. If it were agreed that some easy-to-produce version of medical marijuana could be considered by FDA, then its sponsor would face two problems, one much more serious than the other.
The easy problem would be opposition from drug companies with competing products, but aspirin has always held its own against the brand-name NSAIDs, and even within the aspirin business, Bayer has made no serious attempt to drive the cheaper brands from the field.
The more serious problem is that if marijuana were approved, then the original sponsor would have no effective way of making any money from it. This is the reason why newer uses of aspirin are rarely studied.
You ask if there are any significant differences between the FDA and the UK’s Medicines and Healthcare products Regulatory Agency. The main difference is that the FDA is much bigger, so FDA is the only drug-regulating agency in the world able to look at raw data instead of working from the summaries provided by academic experts. Also, there are at least a few deep thinkers at FDA, but in my experience I came across only one at any other drug-regulating agency, and she was from the German agency, not the UK.
Some other agencies (notably that of Australia) have arms that look at cost-effectiveness, unlike FDA. Some (notably that of Germany) have arms that look at various folk medicines (homeopathy, etc.) by different standards.
FDA Approval as a Botanical