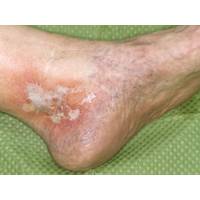
By O’S News Service October 1, 2013
The International Association for Cannabinoid Medicine met in Cologne, Germany, Sept. 27-28. We will be reporting on it in the upcoming print edition. In the photo at left, Raphael Mechoulam is recounting a hypothesis, published by Pacher and Kunos in FEBS (The Journal of the Federation of European Biochemical Societies) in April of this year: “modulating endocannabinoid activity may have therapeutic potential in almost all diseases affecting humans…”
Tod Mikuriya, MD, posited essentially the same hypothesis in 1996, based on a review of the medical literature and histories he’d taken from patients at the San Francisco Cannabis Buyers Club. See Lab Studies by Cannabinoid Researchers Help Explain Clinical Efficacy in O’Shaughnessy’s Spring 2006. In fact, it was Tod’s approval of Cannabis as a treatment for a wide range of conditions that brought down on him the scorn of the U.S. government in 1996. Four Star Drug Czar Barry McCaffrey in 1996 sound-bited that Tod practiced “Cheech and Chong medicine.”
When the name of the game is Medical Science, pharmacology is not the only route to the truth (although as “hard science,” it commands more respect). Mechoulam deserves and may get the Nobel Prize. Mikuriya deserves a posthumous Lasker.
Another IACM presentation that Tod anticipated, in a sense, was the poster by Geitzen et al, “Cannabis in the Third Age: Experimental Therapy at a Nursing Home.”
Geitzen and co-workers studied 19 elderly patients medicating with Cannabis at the Hadarim nursing home at Kibutz Naan in Israel. Patients’ ages ranged from 69 to 101; their disorders included pain, lack of appetite, muscle spasms and tremors. Tikun Olam made three strains available for use by inhalation or oral ingestion as a powder: high THC (23%), THC-CBD (12%:12%), and CBD-rich (16%:1%). The researchers and the Hadarim staff “monitored participants for signs of improvement, as well as improvement in overall life quality, such as mood and ease in completing daily living activities, sleep and the use of prescribed medications.”
Co-author Zach Klein, explaining the poster in Cologne, said : “The big problem is that they can stop using pharmaceuticals. Zyprexa is not supposed to be given to the elderly with dementia because it increases mortality. But that’s what they give for dementia in all the other nursing homes.” The poster featured a matrix listing the adverse side-effects of conventional pharmaceuticals given to the elderly for dementia at other nursing homes that was very reminiscent of one Tod included in his great paper, Cannabis for Post-Traumatic Stress (O’Shaughnessy’s, Spring 2004). Cannabis enables patients to cut down or eliminate the use of conventional pharmaceuticals. Both the Israeli researchers and the California clinician recognized the inherent dangers of “polypharmacy.” 
Yet another IACM poster was foreseen, in a sense, by Mikuriya. It linked Rimonabant to epileptic seizures. Rimonabant is a cannabinoid-antagonist drug marketed by Sanofi for weight loss. It was approved by European regulatory authorities in 2006 but had to be withdrawn because it induced “suicidality.” Tod had written a letter to the U.S. Food and Drug Administration urging that they not approve Rimonabant because any drug blocking the CB1 receptor would very likely cause a wide range of adverse effects (not just mood-related ones). That letter, he felt, may have been taken seriously. The thousands of deaths caused by FDA-approved Vioxx may have made them receptive to his input, at least for a minute.