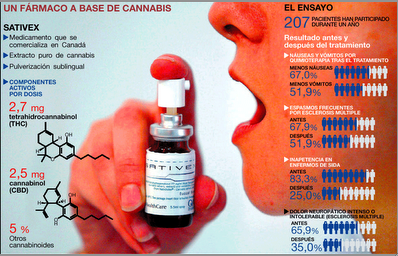
“Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial,” by R.E. Cooper and colleagues at Kings College London has been e-published in European Neuropsychopharmacology.
“Adults with ADHD may represent a subgroup of individuals who experience a reduction of symptoms and no cognitive impairments following cannabinoid use,” the abstract concludes. “While not definitive, this study provides preliminary evidence supporting the self-medication theory of cannabis use in ADHD.” (Thanks to Joe. D. Golstrich, MD, for forwarding.)