The Cannflavins Unique to Cannabis
By Fred Gardner (Advised that pdfs are not the best format for online viewing, we’re creating pages for O’Shaughnessy’s articles of ongoing relevance.)
Flavonoids are compounds produced by many plants that influence the color of flowers, among other things. Flavonoids are defined by a 15-carbon backbone that includes two phenyl groups. Like terpenoids, they are “secondary metabolites,” advantageous to the plant (attracting a pollinator, inhibiting growth of a mold, etc.) but not “primary” components like the proteins, lipids, and carboydrates needed for life itself.
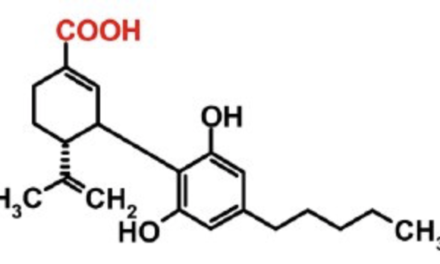
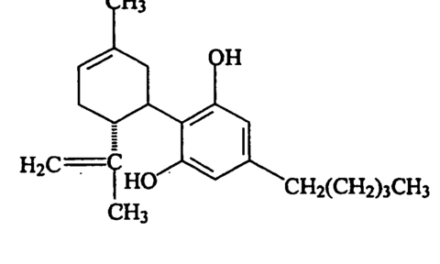
In the 1980s, Dr. Marilyn Barrett identified two diprenylated flavonoids in Cannabis which were previously unknown. She named them “Cannflavin” A and B. In 2013 Mahmoud ElSohly and colleagues at the University of Mississippi identified a third, Cannflavin C.
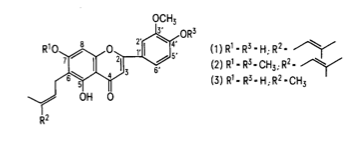
Cannflavin A (structure 1) and cannflavin B (structure 3) were isolated from Cannabis in the 1980s by Barrett et al. These are prenylated flavones—compounds that have a prenyl group (3-methyl-but-2-en-1-yl) attached to their flavonoid backbone. Cannflavin B is different from A, lacking the five carbon alkyl unit at C-4. (Structure 2 was included for purposes of structure identification; it is not a compound found in Cannabis.)
Cannflavins are now being studied for anti-inflammatory activity, and hemp cultivars with unusually high cannflavin content (c. 2%) are being grown in Italy.
We sought some background info from Barrett, who is based in Mill Valley. She describes her discovery as “classic pharmacognosy.” She was a PhD student at the School of Pharmacy, University of London, looking for compounds that would counter the activity of an inflammatory mediator, prostaglandin E2 (PGE2), present in synovial cells cultured from the knee joints of patients undergoing surgery for rheumatoid arthritis.
Barrett and her co-workers found that a cannabinoid-free alcohol extract of Cannabis was inhibiting the release of these inflammatory prostaglandins from the cultured cells. To determine which component of the extract was having the anti-inflammatory effect, they divided the extract into fractions (using preparative thin layer chromatography) and measured the activity of each fraction in the cell culture assay. The most active fraction, in turn, was divided into fractions and its most active fraction selected, and the process repeated until a pure compound was isolated.
“Once you get down to a pure compound,” Barrett explains, “then you can work on identifying the structure of that compound. We used mass spectrometry (MS) to measure the molecular weight along with proton- and carbon- nuclear magnetic spectroscopy to get a picture of the structure. Ultraviolet spectroscopy confirmed we were working with a flavonoid, belonging to the class of flavones
Previous work by Barrett’s colleagues (Fairbairn and Pickens) at the School of Pharmacy used a model of catalepsy in mice to measure the psychoactive properties of the cannabinoids, particularly THC. They were extracting the cannabinoids from dried plant material using petroleum spirit until the remaining plant material was cannabinoid-free. The spent plant material was then extracted with alcohol.
Fairbairn and Pickens determined that this alcoholic extract of Cannabis, which was free of cannabinoids, had the ability to counteract the cataleptic activity of THC in mice. They suspected that the inhibition of prostaglandins was important to this effect, and in confirmation of this idea, inhibitors of cyclooxygenase also demonstrated this activity in mice. It was this work that led to Barrett’s search for an anti-inflammatory agent in the alcoholic extract.
Barrett first published her account of isolating Cannflavin in Biochemical Pharmacology, June 1985. Details of the structure elucidation were published in a second paper in Experientia 42, April 1986.
Although Barrett’s team found Cannflavin to be 30 times more potent than aspirin as an anti-inflammatory in the cell culture assay, it was 18 times weaker than Indomethacin —which is perhaps why no effort was made to develop Cannflavin as a drug.
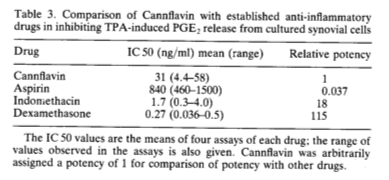
“Especially interesting, from a scientific point of view,” Barrett notes, “might be that the Cannabis plant contains substances that both cause and reduce a cataleptic effect in mice.” This finding was duplicated in the synovial cell assay, in which the cannabinoids stimulated the production of PGE2 and Cannflavin had an inhibitory effect.

Paper by Marilyn Barrett and colleagues in “Biochemical Pharmacology,” June 1985 described the isolation of Cannflavin, the first in a new group of diprenylated flavones.