Yesterday we noted GW Pharmaceuticals’ recently awarded patent of
“an active pharmaceutical ingredient (API) which comprises or consists essentially of cannabidiol (CBD) and one other cannabinoid selected from cannabigerol (CBG), cannabigerolic acid (CBGA), cannabidiolic acid (CBDA), and tetrahydrocannabivarin (THCV) for use as a medicament, and more particularly, for use in the treatment of cancer.”
Kevin McKernan of Medicinal Genomics comments:
This is pretty broad. The supporting evidence is only cell lines and the references cited have some key papers omitted.I’m surprised this passed the novelty test or the obviousness test given the work on CBG and PC3 cells, CBGA and Cox-2 and a host of other papers on Cannabinoids and cancer. Filed in 2015. Thats pretty late for this kind of work.The claim has an API specification which implies they are trying to own the higher purity cannabinoids. A few % terpenes might be enough to cut this. Looks like it probably conflicts with the recent UCANN patent. Wonder which has the earlier priority date?
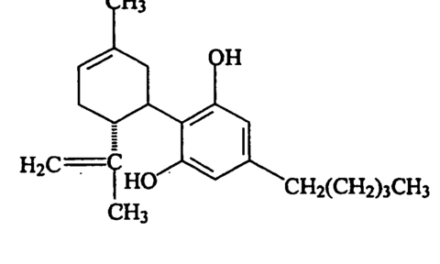
Very broad cannabis patents are now issuing. Any plants that make CBDA and THCA are now covered by a patent issued to the Biotech Institute. These are called Type II Cannabis plants.
Note the broad nature of the claim. Anything more than 1.5% cannabinoids is theirs. The market is filled with 15%-20% cannabinoid plants. If you can’t breed this, you are a dunce. If you can’t get more than 1% terps, you are less of a dunce but that level is hardly novel.
A ratio of 1-to-4 THC:CBD is not novel. It’s arguably the natural state of the plant —but the Patent Office is too ignorant on the topic to examine this and they have very little motivation for accuracy. They make money on re-examinations.
Many people claim to have Type II plants still alive today that predate the 2013 filing of the patent. [THE GRAPHIC SAYS FILED DECEMBER 2015]
Humans bred toward Type I plants during prohibition. Type I plants synthesize all THCA and little-to-no CBDA. Fortunately, we had published the genome of a Type I plant (LA Confidential) in 2011 to assist in “prior-art” generation and public ownership of Type I plants.
By making the sequence of a Type II plant public, we can help enable the community to sequence their documented Pre-2013 Type II plants. CHECK YEAR They can then compare their sequence to this public reference to document the Bt:Bd allele (mentioned in some of the claims) and any other similarities. This will assist in building a Prior Use Exemption and more public evidence for the invalidity of the claims.