The journal Epilepsy & Behavior has just published a special issue entitled “Cannabinoids and Epilepsy” that includes a paper by Drs. Dustin Sulak, Russell Saneto, and Bonni Goldstein, “The current status of artisanal cannabis for the treatment of epilepsy in the United States.” Here’s the abstract:
The widespread patient use of artisanal cannabis preparations has preceded quality validation of cannabis use for epilepsy. Neurologists and cannabinoid specialists are increasingly in a position to monitor and guide the use of herbal cannabis in epilepsy patients. We report the retrospective data on efficacy and adverse effects of artisanal cannabis in Patients with medically refractory epilepsy with mixed etiologies inWashington State, California, and Maine. Clinical considerations, including potential risks and benefits, challenges related to artisanal preparations, and cannabinoid dosing, are discussed.
Results: Of 272 combined patients from Washington State and California, 37 (14%) found cannabis ineffective at reducing seizures, 29 (15%) experienced a 1–25% reduction in seizures, 60 (18%) experienced a 26–50% reduction in seizures, 45 (17%) experienced a 51–75% reduction in seizures, 75 (28%) experienced a 76–99% reduction in seizures, and 26 (10%) experienced a complete clinical response. Overall, adverse effects were mild and infrequent, and beneficial side effects such as increased alertness were reported. The majority of patients used cannabidiol (CBD)-enriched artisanal formulas, some with the addition of delta-9-tetrahydrocannabinol (THC) and tetrahydrocannabinolic acid (THCA). Four case reports are included that illustrate clinical responses at doses b0.1 mg/kg/day, biphasic dose–response effects, the use of THCA for seizure prevention, the use of THC for seizure rescue, and the synergy of cannabinoids and terpenoids in artisanal preparations.
Only yesterday, we demanded that the medical establishment “Acknowledge the Clinical Evidence!” (See below.) And now we’re citing Sulak D, et al, The current status of artisanal cannabis for the treatment of epilepsy in the United States, Epilepsy Behav (2017), http://dx.doi.org/10.1016/j.yebeh.2016.12.032
“Artisinal Cannabis” is a nice, respectful way to describe the range of products being used by patients.
Yesterday’s Note to the SCC with Link to Tashkin on COPD
A report called The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research, released in January by the National Academies of Sciences, Engineering, and Medicine (NAS), was the subject of a “Stakeholders Engagement Meeting” streamed from the NAS building in Washington, DC yesterday.
The authors of the report were evaluating what they called the “strength of the ‘evidence'” regarding the health benefits and risks of cannabis as medicine. By far the strongest evidence, from their perspective, are randomized, placebo-controlled clinical trials (RCTs). Clinical evidence —the findings and observations of physicians who monitor cannabis use by patients— is ignored completely. It’s not even “low quality!”
Retro Messages:
1. The number-one finding makes cannabis use at a young age seem causal when in fact it is a response to an environment (family, neighborhood, school) from which the young user seeks relief.
2.Women who experience nausea during pregnancy tend to smoke more and eat less. The pot-users’ babies gain weight fast and in about a month have caught up with the abstainers’ babies. CITATION NEEDED
3. Smoking marijuana does not exacerbate COPD, according to the great UCLA pulmonologist Donald Tashkin. (I haven’t followed the literature since covering this talk by Tashkin.)
4. The positive finding that “Some people with chronic pain, muscle spasms from multiple sclerosis, or nausea and vomiting from cancer chemotherapy obtain some relief of their symptoms from using cannabis-based products or cannabis” seems like grounds for moving the herb off Schedule I. This is the irrefragable good news in the NAS Report.
5. The demand of the reform honchos is (and has been for many years): “Remove the barriers to research!” They come on like they’re fighting the good fight but they’re just playing their part in the stall. A meaningful demand would be: “Acknowledge the clinical evidence!”
She Saw The Light
 Dr. Staci Gruber, the neuroimaging specialist at Harvard Med School (McLean Hospital) who became a darling of NIDA (the National Institute on Drug Abuse) when she decried the impact of marijuana on the developing brain, surprised us by discussing a pilot study called “Splendor in the Grass?” (Puns in titles and headlines reveal that the author knows the herb is relatively harmless. They would never make light of meth or heroin.)
Dr. Staci Gruber, the neuroimaging specialist at Harvard Med School (McLean Hospital) who became a darling of NIDA (the National Institute on Drug Abuse) when she decried the impact of marijuana on the developing brain, surprised us by discussing a pilot study called “Splendor in the Grass?” (Puns in titles and headlines reveal that the author knows the herb is relatively harmless. They would never make light of meth or heroin.)
Gruber’s team studied new users after three months of “MMJ treatment.” They found “some improvement on measures of executive functions… reduced sleep disturbance, decreased depression, attenuated impulsivity, positive changes in quality of life… a notable decrease in their use of conventional pharmaceutical agents (42% reduction in opioid use).”
As her point of view shifts, is Gruber’s brain undergoing some small structural changes? Are they temporary or permanent? Has her amygdala changed color?
The neoprohibitionist POV was represented by A. Eden Evins, an Addiction Specialist at Harvard Medical School and an advisor to Smart Approaches to Marijuana, the neoprobe front group. (A. Eden is a woman but I flashed on Herb Caen’s line, “Never trust a man who parts his name on the right.”)
Evins criticized the methodology of studies cited by the NAS Report that showed benefit. She noted that some trials “Enrolled only experienced cannabis users (compromises blind, minimizes adverse effects).” Donald Abrams, MD, put in that he had conducted such trials intentionally because people who don’t know how to inhale skew the results in a test of inhaled medicine.
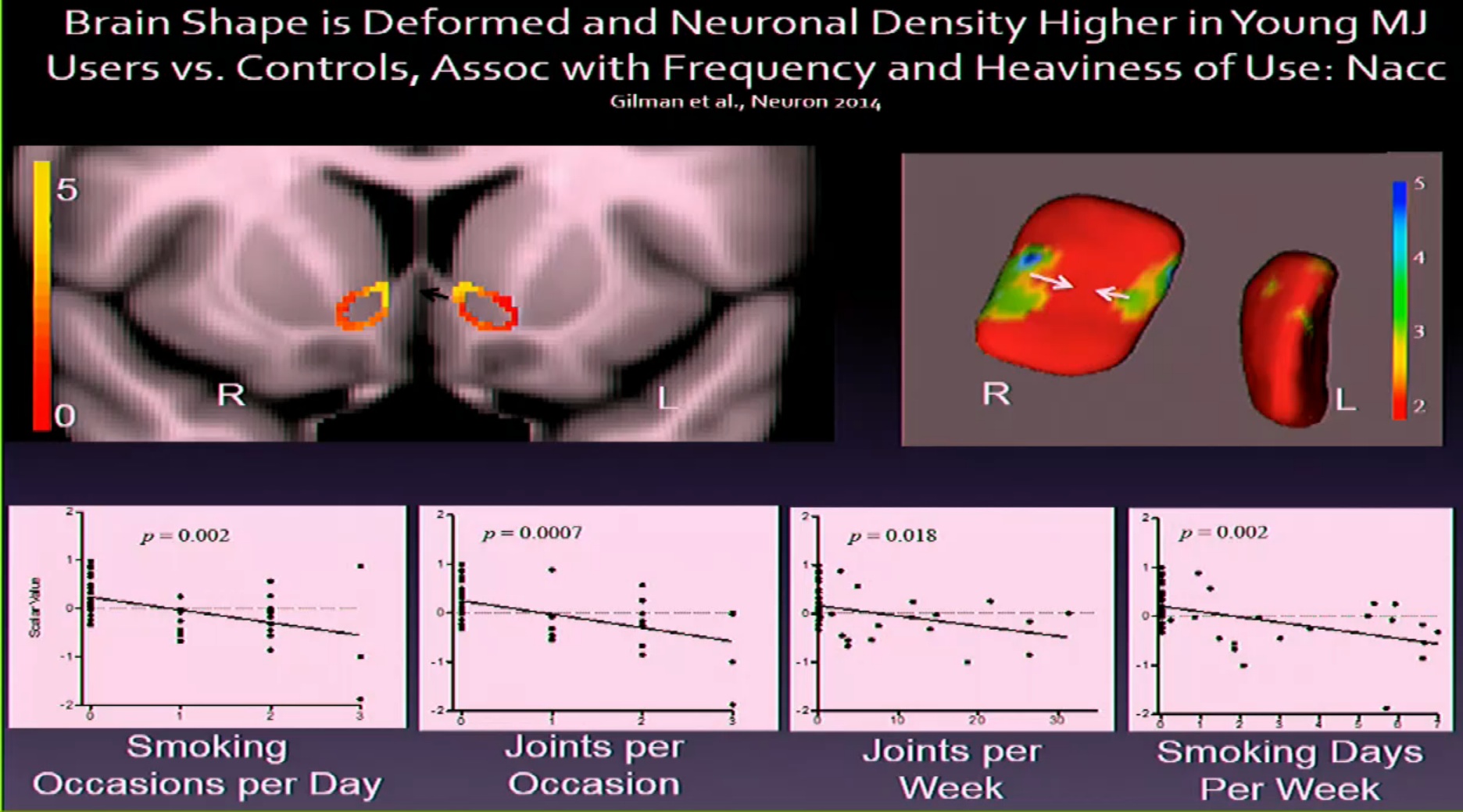
Evins provided the inevitable computer-generated “brain damage” graphic. I think the fried egg was scarier. —Fred Gardner